Introduction
The skin is one of the most exposed organs to detrimental environmental conditions. Sunlight, smoke or air pollution,1 microorganisms, and chemical challenges like household detergents amongst other influences challenge the health of the skin.2 Besides these extrinsic factors, e.g., photoaging processes, the condition of the organ is affected regularly by pure intrinsic aging associated with a reduced number of blood vessels, especially in the upper dermis, which allow diffusion of nutrient rich plasma. Consequently, regenerative abilities of the skin - our first barrier - are affected by increasing age.3–5 Moreover, the barrier between the body and the environment is traumatically injured by wounds caused by various incidents. Due to the initial great regenerative potential of the skin a lot of the integrity losses are cured by proliferation and differentiation of the cellular elements of the skin.6,7 Wound healing can be divided into several phases such as inflammation, proliferation, and remodeling and age-related changes in wound repair have been described in each of these phases.8–10 The underlying mechanisms in folates remain unclear. Skin keratinocytes from older donors have a more limited replicative lifespan than keratinocytes obtained from younger individuals when placed in culture.11,12
Not exclusively, but with increasing importance in the age there are also metabolic dietary factors that may affect skin conditions. Among the most important nutrients for human health, the family of folates plays a key role for human skin. Folates are required in DNA and RNA metabolism, nucleotide, neurotransmitter and erythrocyte synthesis and are important for quickly dividing cells (e.g., mucosa, tissue, hematopoietic system) as well as in many biochemical methyl donation reactions. Furthermore, there are possible links between folate deficiency and disease of the skin like psoriasis, vitiligo, exfoliative dermatitis, glossitis, and skin cancers.13 Accelerated skin proliferation in psoriasis compared to normal could cause a local folate deficiency.14,15
In addition to the metabolic benefits of orally taken folates, it would be interesting to understand, whether folates can cross the skin, providing an alternative route of entry while allowing folates to be applied directly to areas of increased needs.
The disadvantages associated to folic acid use, particularly for topical applications, are the limited solubility and the sensitivity to UV rays.16 The very low solubility of folic acid in aqueous physiological solutions (1.6 µg/ml)17 results in insufficient homogeneous dispersion in hydrophilic solvents. Therefore, surfactants, co-surfactants or co-solvents are required to obtain a homogeneous formulation. The insolubility in organic solvents and the low lipophilicity of folic acid hinders the penetration of the skin while applying penetration enhancers may impair the protective function of the skin barrier.
Since a long time, folic acid is used as food supplement. Folic acid is a synthetic form of the vitamin which is not present in natural food but in fortified foods, supplements and pharmaceuticals. It lacks coenzyme activity and must be reduced to the metabolically active tetrahydrofolate form within the cell.18
We investigated reduced folate salts or formulations with improved solubility characteristics regarding their ability to penetrate the skin in aqueous solution.
Reduced folates like L-formyltetrahydrofolate calcium (L-FTHF Ca) have a limited solubility in water of approx. 1% (10 mg/ml). The solubility properties of L-FTHF Ca significantly improved in a liquid formulation[1] containing sodium gluconate so that precipitation of the folate could be avoided and concentrations of up to 10% (100 mg/ml) could be achieved. The reduced folate salts L-FTHF di-arginine[2] and L-MTHF dicholine[3] are more lipophilic, exhibit good stability, and are at least moderately soluble in organic media, increasing the formulation possibilities for local applications. Furthermore, L-FTHF di-arginine is highly soluble in water. The low solubility of L-methyltetrahydrofolate calcium in water (ca 10 mg/ml)19,20 is also considered inappropriate, while L-MTHF dicholine salt was readily soluble in water (<1 g/ml).
We used human reconstructed epidermal models to investigate the penetration of the selected folates. They are well established means for the evaluation of compounds corrosive or irritant to human skin.21–23 Reconstructed from normal human keratinocytes of healthy donors, they closely mimic the barrier function of normal human skin. Results obtained with these models are validated bases for the classification of chemicals. Moreover, the same models are applied in bioavailability studies in vitro,24–26 because they provide a readily available tool for uptake studies on the human skin without laborious in vivo studies with human volunteers.
The aim of the investigations was to find out whether the selected folate salts penetrate the skin, which is a prerequisite for bioavailability, and whether there are differences in penetration rates between the different formulations. To address at least one aspect of topical application, the effects of these specific folate salts on wound healing were evaluated.
Material and Methods
Chemicals
Preparation of solutions
Solutions for the assays were prepared at room temperature without pH modification as follows: L-FTHF Ca 2.5% (w/w) and sodium gluconate 2.5% (w/w) in ultra- pure water (MilliQ Reference A+, Merck, Darmstadt, Germany) corresponding to 48.69 mmol/L L-FTHF. L-MTHF dicholine or L-FTHF di-arginine 2.5% (w/w) in ultra-pure water corresponding to 37.55 mmol/L L-MTHF or 30.42 mmol/L L-FTHF. Control solutions of 2.5% folic acid in water or 2.5% L-formyltetrahydrofolate calcium in water cannot be used due to the low water-solubility of the compounds.
Experimental Designs
Penetration investigations on skin models
Penetration of folates in skin was evaluated using reconstructed epidermal skin models (epiCS, Henkel AG & Co. KGaA, Düsseldorf, Germany) generated from normal primary human keratinocytes. These models show a barrier very similar to human skin, and are an accepted model for regulatory purposes in skin irritation and skin corrosion tests (e.g., OECD TG 439).21–23
In the keratinocyte skin model the cultured cells form a two-compartment system on a porous membrane. To assess skin penetration of the folates, their solutions were applied to the upper compartment above the keratinized layer. Samples were taken from the lower compartment at defined time points. Although the epidermal skin models are accompanied with quality control data of the respective batch, the cellular barrier was checked for leaks before use by means of trans-epithelial electrical resistance measurements (TEER, EVOM with STX3 electrodes, World Precision Instruments, USA) to determine the applicability of the epidermal models at the study site. Suitable epidermal skin models we treated topically with 50 µL of the test product solutions prepared as described under “chemicals” without further dilution or with water as control. The basal compartment at the bottom was filled during treatment with 1000 µL HBSS (Hanks balanced salt solution, Lonza, Switzerland) to exclude influences of the complex and proprietary cell culture medium on subsequent analyses. After 4h, 8h (data not shown), and 24h, samples of the buffer solution (500 µL each) were taken from the basal compartment below the membrane and checked for presence and concentration of folates. 500 µL of fresh HBSS was added after 4h and 8h to the basal compartment, to replace the volume removed. Each treatment was tested with three models in two independent runs (n=6 in total). Topically applied water served as control.
Concentrations of folic acid, L-FTHF and L-MTHF in buffer samples were measured by a HPLC-MS/MS method (limit of detection = 0.2 nmol/L folic acid, 0.09 nmol/l L-FTHF, 0.1 nmol/l L-MTHF), established by the University of Saarland, Germany.27
Subsequently to the topical treatment for 24h, the epidermal models were washed and incubated with the vital dye Resazurin (Merck Millipore, Darmstadt, Germany) to determine the relative number of living cells in the models. After 2h incubation, samples of the basal medium were analyzed on Resarufin, the redox product of Resazurin, formed by vital cells by measuring the fluorescence at 560/590 nm on a fluorescence reader (Infinite M200 pro, Tecan, Austria).
Wound healing assay
To examine the effect of different reduced folate preparations on wound healing an in vitro scratch assay was used. This in vitro model comprises the defined injuring of a closed cell layer and the subsequent recovery of the defect in the presence of a test substance compared to appropriate controls, i.e., (untreated) cells with no test substance present.
In preparation to the scratch assay, different concentrations of the test chemicals were applied to confluent monolayers of primary keratinocytes to find applicable dosages. For that, primary keratinocytes of human skin (C-12005, Promocell, Heidelberg, Germany) were cultured in the medium recommended by the manufacturer (C-20011, Promocell, Heidelberg, Germany). Keratinocytes were seeded on 96-well plates cultured to confluence and incubated with five dilutions of the folate solutions for 24h. Methylthiazolyldiphenyl-tetrazoliumbromide (MTT) (MTT, 0.5 mg/mL M2128, Merck, Darmstadt) was added for 2h and the color change caused by the conversion of the vital dye in living cells was quantified photometrically at 590 nm. Concentrations resulting in relative viabilities of more than 70% compared to the control (see below) qualify for the scratch assay. Three different non-toxic dosages of the folates were used in the scratch assay.
For the scratch assay, keratinocytes were cultivated in appropriate cell culture plates, (6-well plates, Greiner BioOne, Frickenhausen, Germany) bearing position markings on the outer bottom of each well, until they completely covered the growth area. Removing cells with a pipette tip along the position marking generated an area free of cells representing a scratch or injury. After rinsing the cell culture plates with phosphate buffered saline to remove partially detached cells, the plates we covered with medium comprising the test-substances in appropriate dosages. Regrowth of the cells into the cell free area was documented photographically at 0h, 6h and 24h. Analysis was done by marking and calculating the cell-free area using the software ImageJ.28 The relative closure of the wound area was calculated by subtracting the relative wound area at each time point from the initial wound area (=100%). For every dosage of each test substance, two replicates were treated in a single experiment. As control served standard serum free medium without folate and standard medium added with 10% fetal bovine serum (FBS, Biochrome, Berlin, Germany).
Results
Penetration and metabolism
During the 24h contact period, the topically applied folate derivatives penetrated the epithelium of the reconstructed epidermal skin models. The solutions of the lower compartments of the epidermal skin models were analyzed by LC-MS in order to detect both the penetration and metabolism of folic acid, L-MTHF and L-FTHF.
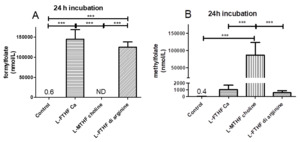
Folic acid concentrations in all samples were between 20 nmol/L and 60 nmol/L which are irrelevant low concentrations for assessing the metabolism of the novel folate salts (data not shown). L-MTHF and L-FTHF concentrations increased during the testing period and were found in micromolar concentrations.
After 24h, a concentration of 145.2 µmol/L L-FTHF was determined in the skin models treated with L-FTHF di-arginine a concentration of 125.2 µmol/L was found (Figure 2A). The addition of L-MTHF dicholine to the skin models resulted in the detection of a concentration of 86.2 µmol/L L-MTHF after 24h (Figure 2B). Remarkably, the models treated with L-FTHF Ca and L-FTHF di-arginine also showed increasing concentrations of L-MTHF (L-FTHF Ca: 155 nmol/L after 8h, 1018 nmol/L after 24h; L-FTHF di-arginine: 76 nmol/L after 8h, 554 nmol/L after 24h), but significantly lower concentrations than after treatment with L-MTHF dicholine (29.8 µmol/L after 8h, 86.2 µmol/L after 24h).
The differences in L-MTHF concentrations in the models between the models supplemented with L-FTHF Ca and L-FTHF di-arginine on the one hand and the L-MTHF dicholine supplemented skin models on the other hand were highly significant. The skin models treated with L-FTHF Ca had the highest concentration of L-MTHF (1118 nmol/L) after 24h. The concentration of the solution below the models treated with L-FTHF di-arginine was approximately half as high (620 nmol/L). L-FTHF was not detected in the samples after application of L-MTHF dicholine because L-FTHF cannot be formed from L-MTHF by human cells or only by energy-consuming metabolic pathways.
The viability of the epidermis models was not adversely affected by treatment (data not shown). A slight decrease in viability (86% of the control) was observed after treatment with L-MTHF dicholine. However, a decrease of this magnitude is not considered a toxic effect. For comparison, in skin epidermis models treated with potential irritants, viabilities greater than 50% of the untreated control are considered non-irritant.21
Wound healing assay (scratch assay)
The results of the dose finding experiment before the scratch assay are shown in Figure 3. As suitable highest dose for the scratch assays the following concentrations were chosen: 1.5 mmol/L for L-MTHF dicholine, 1.95 mmol/L for L-FTHF Ca and 1.22 mmol/L for L-FTHF di-arginine (Figure 4).
After 6h, there was no substantial coverage of the cell-free area in all treatment groups. After 24h, 50 to 77% of the initial wound area was covered. At this point, the folate solutions tended to promote growth better than the standard medium (Figures 4 and 5). The different folate solutions stimulated growth similarly (60‒77% recovery), while less recovery was observed in the controls (30‒66%).
Addition of the selected folate preparations tended to accelerate the regeneration of an artificial wound after 24h in the scratch assay.
Discussion
Hasoun et al. (2013)29 found low folate concentrations in human epidermis compared with many other tissues. At the same time, they identified a relatively high proportion of L-MTHF in healthy epidermis compared to the dermis. They discuss a special role for L-MTHF in the epidermis with respect to possible photo degradation and maintenance of the high proliferation rate typical for the epidermis. In general, these findings indicate special requirements of the epidermis regarding folates.
To the best of our knowledge this is the first time[4] that penetration of highly potent aqueous solutions of folates, free of allergenic or irritating substances, has been demonstrated in skin epidermis models. The penetration of reduced folate salts through the epidermis and therefore reaching the dermis makes folate probably bioavailable for topically applications. Most impressing, L-FTHF was metabolized partially to L-MTHF, the dominant form in the body and the cellular part of the skin. L-MTHF was metabolized from these two derivatives. After 24h, the L-MTHF concentrations in the samples of the skin epidermis models treated with L-FTHF-Ca were twice as high as after L-FTHF di-arginine treatment. However, it must be taken into account that the applied molarities of the two L-FTHF salts differ by about one third (L-FTHF-Ca = 48.69 mmol/L; L-FTHF di-arginine = 30.42 mmol/L), which contributed to a large part of the difference. After 24h, the difference between the supplementation groups was marginal. The occurrence of metabolites of the added salts underlines the accessibility of the folates for human skin cells despite distinct horny layer. Those findings highlight the physiologic capacity of in vitro reconstructed skin epidermis models, which largely rebuild the barrier function of human skin.
L-MTHF was found in high levels opposite from the treated side of the epidermis models. L-MTHF dicholine seemed to penetrate easily through the keratinized layer and the cell layers below.
The viability of the models was not significantly affected by the treatment. Therefore, we assumed that the barrier function of the epidermis models was not impaired. Compared to the test on irritating effects according to OECD TG 439,21 where models are treated with the test substances for only 15 min, treatment over 24h is far more challenging. Thus, the conclusion might be that there is a very good compatibility to the human skin for the tested folates.
The study has some limitations. Since the reconstructed skin epidermis tissue models as such were not examined on folate content, it is unknown if and to what extend the folates are retained in the reconstructed epidermis tissue. Moreover, it is not clear which specific cell layer of the model is responsible for the metabolic conversion of folates. Future approaches might engage more into the processes within the tissue even integrating dermal elements making use of full thickness skin models.
Humans are not able to synthesize folate de novo, and therefore are dependent upon dietary sources. The terms folate and vitamin B9 refer to a large family of chemically similar compounds different in the number of glutamine residues, type and position of one-carbon substituent or the oxidation state. The most widely known folate is the synthetic folic acid due to its enhanced chemical stability.30 Folic acid is inactive in the body and needs to be converted to reduced folates by several enzymes e.g. in a rate limiting step by dihydrofolate reductase.18,31,32 In further reducing steps, dihydrofolate and dietary folates as monoglutamates, are converted to the bioactive folate form in the body, e.g., L-MTHF. The folate uptake and metabolism is depending on many genetic polymorphisms affecting the status of folate and vitamin B12 resulting in elevated homocysteine.33,34 While folate deficiency has been extensively studied by analysis of human plasma, the folate status in skin has not been widely investigated. Inefficient delivery of micronutrients to skin and photolysis of folates argue that folate deficiencies will be present if not exacerbated in skin.30 Therefore, a targeted delivery of micronutrients might be of future interest to circumvent or reduce systemic impairments. The characteristics of the selected reduced folates as determined here support their applicability with this respect.
In addition to the uptake studies, aqueous solutions of the investigated folates exemplarily showed positive tendencies on wound healing in the scratch assay. This effect of the tested compounds in wound healing provides hints on a possible field of application improving the integrity of deficient epidermal barrier.29
Whether or not non-differentiated skin keratinocytes in monolayer culture are able to transform folates in a similar manner as highly differentiated keratinocytes in skin models have to be addressed in future work. Results obtained from such trials might help to understand which folates are best choice to promote wound healing or treat skin related diseases.
Conclusion
In the epidermal skin model, highly concentrated aqueous solutions of reduced folates (2.5%) successfully penetrated the intact barrier of the epidermal model. Most impressively, the added L-FTHF was partial metabolized to L-MTHF, the dominant form in the body and the cellular part of the skin. The local occurrence of metabolites of the added folate salts underlines the accessibility for human skin cells. Furthermore, the results of the scratch assay with primary keratinocytes indicate improved skin reepithelialization during wound healing. Thus, the proliferation and migration of epidermal cells is supported. In individuals with a disease-related low folate status, a topical folate application may help to improve skin conditions. The epidermal penetration ability of the tested reduced folates, free of any allergenic or irritating substances, opens up new possibilities for the development of topical applications for the local administration of folate.
Conflict of interest
DD and KEJ are working for BioTeSys. CL has no commercial or financial relationship that could be construed as a conflict of interest.
Author Contributions
All authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for the authorship for this article as a whole, and have responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
DD study design, data analysis; interpretation, drafting the manuscript: DD, KEJ and CL. The sponsor had no role in the design, interpretation, data analyses, or drafting of the manuscript.
Funding
The study was funded by Aprofol AG, Appenzell, Switzerland.
Acknowledgments
The authors thank all people that contributed in laboratory work and Aprofol for providing the folate samples.
Patent owner Aprofol AG
Patent owner Aprofol AG
Patent owner Aprofol AG
Data provided as preprint on bioRxiv doi: https://doi.org/10.1101/2021.04.07.438798.
_and_l-formyltetrahydrofolate_di-a.png)



_and_l-formyltetrahydrofolate_di-a.png)



