Introduction
Glaucomatous optic neuropathy includes many morphological changes, from thinning of the nerve fiber layer in the retina to cell loss in the visual cortex.1 Functionally, the deficits are also manifold, including issues ranging from color vision deficits to electrophysiological changes. From a clinical point of view, optic disc excavation and visual field defects are significant.2
The most common risk factor is elevated and/or a highly fluctuating intraocular pressure (IOP), but the visual fields of many glaucoma patients worsen despite well-controlled IOP.3–7 Moreover, the prevalence of normal-tension glaucoma (NTG) is high, especially in Asian countries, and seems to be increasing further. Factors other than IOP are also involved.5,8–11 The most common are vascular factors.8 It has been known for decades that glaucoma in patients with low blood pressure (BP) has a worse prognosis than in patients with normal blood pressure.12 Taking the two factors, BP and IOP, together and calculating perfusion pressure (PP) results in an even better prediction of future progression.13
It was assumed that retinal venous pressure (RVP) would be equal to IOP for a long time. An increase in RVP was expected only in people with elevated intracranial pressure.
The introduction of the ophthalmodynamometer by Löw14 made it possible to measure RVP, which made it apparent that RVP is often higher – sometimes much higher – than IOP in many situations and diseases.15–17 Higher RVP reduces PP and it also induces higher capillary pressure upstream or, in other words, higher transmural pressure.18
Increased RVP was described not only in eyes with retinal vein occlusion (RVO),19 but also in their clinically healthy partner eyes,20 in glaucoma,21 in diabetic retinopathy,22 in people with Flammer syndrome,23 in central serous chorioretinopathy,24 and even in healthy climbers at low-oxygen altitudes.25
Nevertheless, clinical interest in RVP remained relatively limited for a long time, as it was assumed that this increase in RVP was due to mechanical obstruction or compression of the vein and, therefore, not amenable to therapy.
Flammer and colleagues showed that vascular dysregulation can occur not just in arteries but also in retinal veins26 and that local functional constriction of the veins can increase RVP18 and, in extreme cases, even lead to retinal vein occlusion.27 Endothelin and other vasoconstrictors play a central role in this process.18,28–30 Thus, the prerequisite for pharmacological reduction of increased RVP was provided. Subsequently, RVP was shown to be reduced by endothelin blockers,31 calcium channel blockers,32 anti-vascular endothelial growth factors (anti-VEGFs),33 and to some extent even by antioxidants.34 Thus, the desire for preparation with few side effects and preferably no contraindications grew.
In this study, we tested Ocufolin® forte, a multivitamin preparation containing L-methylfolate. Folates play a central role in metabolism, specifically in methylation (C1-unit transfer) of important molecules, such as DNA. Folate deficiency also increases homocysteine (Hcy), which is also often elevated in glaucoma.35,36 Ocufolin® forte has been shown to reduce Hcy in patients with diabetes most efficiently.37
One must note that folic acid and L-5-methyltetrahydrofolate (L-methylfolate, L-5-MTHF) are not the same things.38 The common commercially available (oxidized) folic acid must first be converted into the biologically active form in our body by reduction and methylation. This process is slowed down in the case of frequently occurring polymorphisms of dihydrofolate reductase (DHFR) or methylenetetrahydrofolate reductase (MTHFR). In order to reach the brain, folate must be actively transported through the blood-brain barrier.39
Even with normal levels of folate in the blood, folate levels in the cerebrospinal fluid may be decreased due to autoantibodies against folate receptor alpha or due to mitochondrial dysfunction.40 Increasing the intake of oxidized folic acid, then, not only brings no benefit but may even be harmful.41
The L-methylfolate contained in Ocufolin® forte is already in its biologically active form and is therefore not affected by the aforementioned polymorphisms. In addition, it can be transported through the blood-brain barrier via the “reduced folate carrier”.42,43
The aim of this study was to test whether Ocufolin® forte reduces an elevated level of RVP and decreases Hcy in patients with glaucoma and/or ocular vascular diseases.
Patients and Methods
Patients were included if all of the following criteria were met: presence of glaucoma and/or ocular vascular diseases in at least one eye, abnormal steady-state pattern electroretinography (SSpERG) result, RVP (as measured using the ophthalmodynamometer) at least 15 mmHg higher than IOP at the optic disc margin or within its borders, fasting serum Hcy level >12 µmol/l, and stable and well-controlled IOP with or without IOP-lowering treatment.
Exclusion criteria consisted of starting other systemic or ocular medications with potential impacts on RVP within a month before entering the study or during the course of the study (e.g., calcium channel blockers, magnesium, anti-VEGF), starting or changing the dosage of other medications with potential impact on SSpERG within 3 months before entering the study or during the course of the study (e.g., anti-diabetics, anti-hypertensive drugs), non-adherence to the follow-up schedule, or the inability to perform a proper RVP measurement using ophthalmodynamometry. Patients were asked not to change any other medications or food supplements during the course of the study.
Study Plan: Provided the inclusion criteria were met, RVP measurement was repeated, and a 1 capsule/day regimen of Ocufolin® forte was subsequently carried out for a duration of 3 months, after which repeat measurements of IOP, SSpERG, and RVP were performed, along with dilated fundoscopy. In some cases, a repeat serum analysis, including measuring fasting serum Hcy, was performed after the 3-month visit. Indications for obtaining a new blood sample were any of the following: RVP >15 mmHg higher than IOP in any vessel within the optic disc or at its border, SSpERG not fully recovering, or persistent clinical signs of high RVP during fundoscopy (arteriovenous crossing signs and/or venous dilation or engorgement and/or venous vessel caliber changes and/or clinical picture of branch retinal vein occlusion [BRVO]).
Methods: IOP was measured using Corneal Visualization Scheimpflug Technology (Corvis ST; Oculus, Wetzlar, Germany) to allow for the correction of the biomechanical properties of the cornea.44–48 Ganglion cell function was measured using SSpERG (Nova; Diopsys, Inc., Pine Brook, NJ, USA). Progression was diagnosed by abnormal SSpERG indicating disease activity with or without signs of structural progression on spectral-domain optical coherence tomography (SD-OCT, iVue; Optovue, Fremont, CA, USA) and/or signs of progression on standard automated perimetry (SAP, Octopus; Haag-Streit AG, Koeniz, Switzerland). RVP was measured using the Ophthalmodynamometer according to Löw (Meditron GmbH; Voelklingen, Germany).14,49,50 If anatomy permitted, RVP was measured in the central retinal vein, the hemiveins, and quadrant veins. The IOP was then further increased until the arteries began to pulsate. This eye pressure corresponds to the diastolic pressure in the corresponding arteries (OAPdiast). OAPdiast was measured in all eyes in order to avoid confusion between high RVP and pulsations that can be observed in the venous system at OAPdiast. If a vessel was not exhibiting visible pulsations during ophthalmodynamometry, it could not be included in the analysis. RVP was calculated at all measurement locations by adding IOP to the measured ophthalmodynamometric value. In keeping with the literature, we present this value as ophthalmodynamometric force (ODF).
Statistical Analysis: As appropriate, descriptive statistics are presented as mean ± standard deviation (SD) or counts and percentages. In order to compare mean pre-post RVP, IOP, and Hcy values, linear mixed-effects models were performed. These models are commonly used for repeated measure data. Mixed-effects models consist of random effects and fixed effects as predictor variables. Details are described elsewhere.51
Here the random effect is “Subject”, the fixed effect is “Timepoint” (pre, post). In the case of the dependent variables IOP and RVP, the eyeside is nested within the subject random effect. Results are presented as p-values of the corresponding comparison. Only values measured at the same location immediately prior to initiation of supplementation and after completing the 3-month course were used for RVP calculation. Therefore, of the 24 subjects, 21 were included in the final analysis. For Hcy, pre- and post-values are distributed over a larger timeframe. A p-value <0.05 is considered to be significant for these values. All evaluations were performed using the statistical software R, version 4.04.52
Results
From 65 patients with high RVP and SSpERG abnormalities, 26 patients (16 men, 10 women), with median age 72 years (range: 51 years–87 years), had serum Hcy >12µmol/l and fulfilled the inclusion criteria.
Two of these patients dropped out during the study. Both had primary angle-closure glaucoma (PACG) and had developed a unilateral BRVO shortly before entering the study, and of these, one had been treated contralaterally with scatter laser photocoagulation 17 years before because of proliferative diabetic retinopathy. Both eyes of both patients with BRVO were excluded from the final analysis, as both patients needed supplemental therapy including systemic medications and anti-VEGF treatment during the course of the study.
Of the remaining 24 patients (16 men, 8 women), 19 patients had glaucoma (9 patients with NTG, 4 with PACG, 4 with pigment dispersion glaucoma [PDG], 1 with primary open-angle glaucoma, 1 with posttraumatic glaucoma), 4 patients were glaucoma suspect (2 NTG suspect, 1 PACG suspect and 1 with bilateral mild pigment dispersion syndrome and unilateral chronic Irvine-Gass syndrome) and 1 had bilateral unexplained visual field loss with concurrent bilateral abnormal pERG and unilateral epiretinal membrane.
Of these 24 patients (48 eyes) who completed the study, 5 patients (9 eyes) were under treatment with topical IOP-lowering monotherapy (prostaglandin or beta-blocker) before and during the study. This therapy remained completely unchanged during the study. Optimization of angle anatomy had been done in the past for all cases with PACG or PACG suspect. Also, 9 patients (17 eyes) had a history of selective laser trabeculoplasty (SLT), and 4 patients (6 eyes) had a history of glaucoma surgery. No systemic or local medications were changed in any patient during the study.
As adherence to the capsules was not always perfect, some patients were allowed extra time in order to finish a total of 90 capsules. The interval after initiation of the supplementation thus spanned 90 to 115 days for the “3-month” visit.
Supplementation with Ocufolin® forte was well tolerated by all patients, and we did not notice any side effects. We had the chance to observe RVP in some patients at earlier points in time, where an RVP-lowering effect could already be observed after 2 weeks in some but only occurred between 6 and 12 weeks in others.
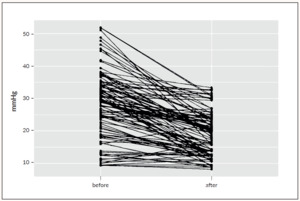
Mean IOP was stable (p=0.15) during the study, and mean RVP dropped by 8.8 mmHg (p<0.001), from 28.1±11.7 mmHg at pre-supplementation to 19.3±7.4 mmHg at 3 months.
When the RVP values averaged for the individual eyes (N=42) are included in the analysis, the level of statistical significance is p<0,001. If the RVP values of the individual eyes per patient (n=21) are also averaged, the level of statistical significance remains at p<0,001.
The RVP and IOP values post- versus pre-supplementation are depicted in Figure 1. The individual course of RVP in 106 vessels of 42 eyes is shown in Figure 2.
In 22 of the 24 patients, serum Hcy was analyzed again between 3 days and almost 41 weeks after cessation of supplementation. The mean serum Hcy level was 15.8±2.8 µmol/l before the start of the study and had dropped to 12.51±2.90 µmol/l (p<0.001, Figure 3).
Discussion
These data show that a 3-month supplementation with a multivitamin preparation containing L-methylfolate can significantly reduce RVP in the setting of glaucoma and/or other ocular vascular disease with a fasting serum Hcy level >12µmol/l. Also, a significant Hcy-lowering effect was confirmed.
This study was undertaken in a group of difficult cases that progressed despite good IOP control. Progession was defined by abnormal SSpERG ± structural progression on OCT ± functional progression on SAP. The magnitude of RVP reduction differs between patients and eyes and depends on factors such as type of disease and location of the measurement. No change in IOP-lowering therapy was made during the study and IOP was stable over the course of supplementation. Thus, this implies that Ocufolin caused an IOP-independent RVP reduction. In an effort to avoid potential impact of systemic diseases or medications on our findings, any start or change of dosage of systemic or ocular medications with known potential effect on RVP or SSpERG was an exclusion criterion and patients were asked not to change other medications throughout the course of the study. It should therefore be safe to conclude the measured differences can be attributed to the intake of the supplementation.
The reduction of Hcy levels observed was smaller than previously reported,37 however, Hcy values at the end of the study were likely to suffer negative selection, as cases with full normalization of SSpERG and concurrent satisfying ODF drop had no repeat analysis. Also, the time delay between ODF measurement at month 3 and the repeat Hcy serum sample afterward may have caused serum Hcy to have already begun rising again, as supplementation ceased in the meantime. Still, Hcy levels sampled after the end of the study were significantly lower than the pre-supplementation levels.
Hcy is an intermediate metabolite produced by demethylation of the essential amino acid methionine.53 Lack of cofactors and/or inborn enzymatic errors can induce accumulation of Hcy.54 As Hcy has many deleterious effects on vascular and ocular health, lowering its serum levels potentially has an impact on different pathways.55–64
In addition to its effect on serum Hcy levels, the studied multivitamin preparation containing L-methylfolate also has antioxidative effects. It is currently unknown which of the constituents or pathophysiologic mechanisms addressed is essential for its RVP-lowering effect. This is the subject of further studies and analyses.
During the course of the study, some clinical observations were of interest.
-
Huge differences in RVP can be noted between different veins and within the same vein over its course from the optic nerve head (ONH) center to the outer ONH border, which fits with the model proposed by Levine and Bebie.65 For this study, we took care to always measure RVP at the same location along a specific vessel. Interestingly, in some eyes, RVP remained high in at least one vein, or fundoscopic signs indicating high RVP were still present in spite of the appearance of spontaneous venous pulsations at the optic disc margin (indicating that RVP at that location had fallen to the level of IOP). A measurement method to evaluate RVP outside the ONH would therefore be of value. Measurement of RVP in different veins per eye can be important in the management of glaucoma, as higher RVP in hemiveins has been associated with worse mean deviation in the corresponding hemifield.17
-
As there is a relationship between RVP and RVO, the presence of 2 cases with BRVO in this series is not surprising.20,27,66 It is disconcerting that in our 2 cases with BRVO, RVP in the affected vessel was not measurable, while a clear RVP-lowering effect was found in the other vessels (data not shown). Also, our observation that the clinical picture in the BRVO area was not improving suggests that RVP in the offending vessel remained elevated over the course of the study.
-
Some patients may benefit from additional monitoring and treatment, as was shown in one of the cases with BRVO who also had vitamin B12 malabsorption and exhibited persistent high Hcy, improving only with additional vitamin B12 supplementation by sublingual tablets.
This study has several limitations. As this is an observational study, no placebo group was established. Only patients with Hcy >12 µmol/l were included, as suggested by the literature.67 However, a recent review suggests that the benefits of a lower cut-off of 11µmol/l should be investigated.68 The complete cohort was treated for 3 months only, irrespective of the obtained serum level under supplementation. This heterogeneous cohort has the advantage of representing a real situation in clinical practice, but the disadvantage that the results cannot be automatically extrapolated to all glaucoma subtypes.
Several important questions remain unanswered. It is still unclear what the target RVP should be to impede further structural or functional damage. SSpERG may offer a method to evaluate the functional consequences of high RVP, and we will report on the evolution of SSpERG values over the course of the study in a separate publication. Our current observations indicate that an RVP-lowering effect can be obtained within 2 weeks in some patients, but as of today, it is unclear within what time frame an RVP-lowering response can be expected and when this RVP-lowering effect is maximal.
In conclusion, Ocufolin® forte had a clinically relevant and statistically significant RVP-lowering effect and led to a clinically relevant and statistically significant Hcy decrease, offering a new treatment modality for eye diseases with increased RVP, such as glaucoma.
Take-Home Messages
-
Retinal venous pressure (RVP) measurement can provide vital information for the management of glaucoma.
-
Elevated RVP can be reduced by targeted vitamin supplementation.
Conflicts of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as potential conflicts of interest.
Author Contributions
Study concept, collection of clinical data, article text: TD; statistical analysis, graphs: AS.
Funding
This study was not supported by any funding.
Acknowledgments
The authors wish to thank Prof. Josef Flammer for his continued support throughout the course of the study. Aprofol kindly offered samples of Ocufolin® forte. Ella Vanpoucke helped with arranging the data tables. This study was registered in ClinicalTrials.gov with registry ID NCT05080153.
_and_iop_(blue_bars)_values_(mean___-_sem)_post_versus_pre-supplementation_.png)
_post-_versus_pre-supplementation.png)
_and_iop_(blue_bars)_values_(mean___-_sem)_post_versus_pre-supplementation_.png)

_post-_versus_pre-supplementation.png)