Introduction
The understanding of glaucoma has changed. This overview article aims to summarize the clinically relevant aspects of glaucoma.
Glaucoma is a relatively common disease that, if left untreated, can lead to visual field defects and even blindness. The most significant risk factors are increased intraocular pressure and impaired regulation of ocular blood flow. Activation of the glial cells and oxidative stress in the axonal mitochondria play an important role. Both are the result of an unstable oxygen supply. Diagnostics focus on both glaucoma damage and the risk factors that cause this damage. Therapy is based on the risk profile. In most cases, reducing the intraocular pressure is the primary approach. If, despite this, the condition progresses, then further treatment is indicated.
Definition of glaucoma
No uniform definition of glaucoma exists in the current literature. The phenomenological definition is the most commonly used one today. According to this definition, we speak of glaucoma if atrophy of the optic nerve head (ONH)
-
is accompanied by excavation of the ONH;
-
shows gradual progression; and
-
leads to visual field defects.
Therefore, glaucoma is a condition with several risk factors.1,2
Phenomenology
Glaucoma damage, most commonly called glaucomatous optic neuropathy (GON), has both morphological and functional aspects.
Morphology
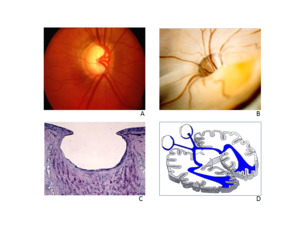
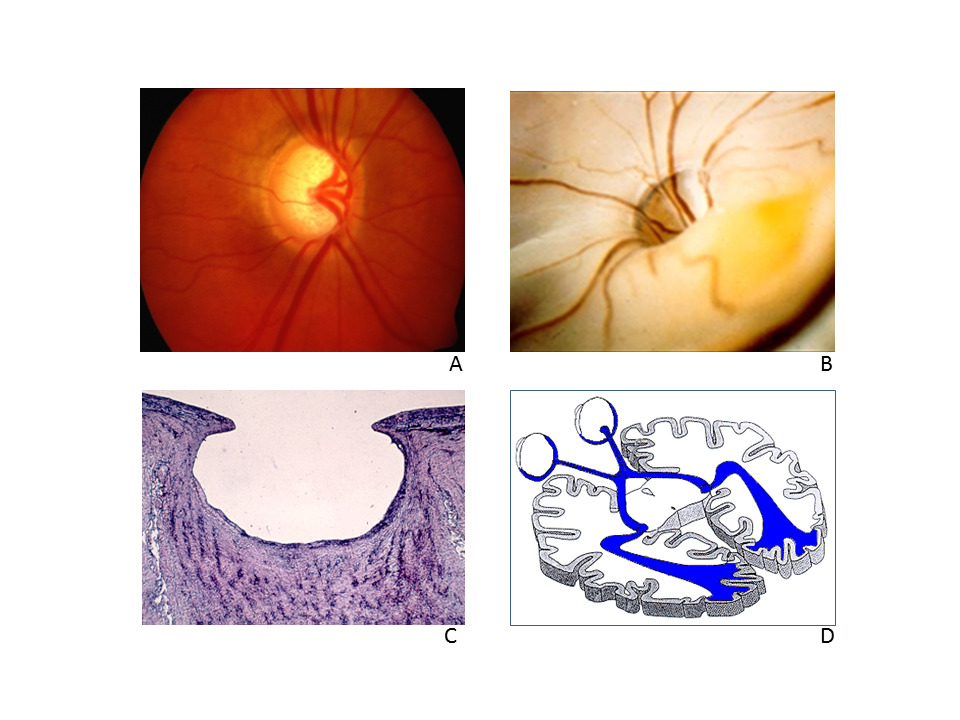
GON has two main components: loss of retinal and neuronal ganglion cells and tissue remodeling, which in turn leads to ONH excavation. Neuronal cell loss, although the decisive factor in the loss of function, is non-specific. However, tissue remodeling, which in turn leads to ONH excavation, is specific. The reason for this lies primarily in the cell death of the astrocytes. In cases of non-glaucomatous lesions, for example, after anterior ischemic optic neuropathy (AION), the glia not only survives but proliferates, which causes glial scarring. GON is clinically identified primarily by excavation of the ONH. Based on pathological-anatomical studies and magnetic resonance tomography (MRI) examinations, we now know that the entire optic tract extending to the visual cortex is affected in a way and to an extent that cannot be explained solely by transsynaptic atrophy (Figure 1).
Function
GON causes various psychophysical deficits. During clinical diagnostics, searching for visual field defects (scotomas) using perimetry has proven to be effective. Glaucoma-related visual field defects are often illustrated as black spots or bars. However, this does not correspond with the perception of glaucoma patients. In the same way that we do not perceive our blind spots, glaucoma patients either do not perceive their blind spots or only do so when they are at a very advanced stage. The reason for this is the way information is processed in the brain, which uses interpolation to fill in the missing information.
Pathomechanisms of GON
Cell damage
As stated above, glaucoma differs from other types of optic nerve diseases in the behavior of the glia. The astrocytes change their morphology and gene expression. If these cells lose their normal structure, the light scattering increases, which can be observed in the retina using red-free light. The transport of oxygen to the axons primarily takes place via the intracellular hemoglobin system of the astrocytes. If the cell offshoots partially lose contact with the axons or capillaries, then the axons suffer from hypoxia, even when the blood supply is still intact. The increased nitric oxide synthase causes an increase in nitric oxide (NO) levels, which – despite the short half-life – can diffuse into the adjacent axons. If the mitochondria are simultaneously producing increased levels of superoxide due to an unstable oxygen supply, NO fuses with superoxide and creates harmful peroxynitrite. Although astrocytes usually have a good survival rate following hypoxia, they are particularly sensitive to oxidative stress and eventually die off following an activation phase.
Oxidative stress
Oxygen free radicals are also produced under physiological conditions and can subsequently be removed by our bodies.
The disruption of this equilibrium results in oxidative stress. This stress damages molecules that either need to be repaired (DNA repair) or subjected to target elimination (protein degradation by proteasomes). If these mechanisms become overstretched, then there is a build-up of damage. Oxidative stress can manifest locally in individual cells or even in cell organelles, such as mitochondria.
Myelinization of retinal ganglion cell axons begins beyond the lamina cribrosa. The myelin sheath is missing in the retinal nerve fiber layer to allow the retina to remain transparent; it is also missing in the papilla because over one million nerve fibers exit the eye via the narrow sclera canal and the pores of the lamina cribrosa. These non-myelinated fibers require a great deal of energy, so they contain a large number of mitochondria. Simultaneously, this tissue is exposed to exceptional stress, since a) the axoplasmic transport must overcome a pressure gradient, b) the axons and mitochondria are directly exposed to light, and c) the blood supply in this area is especially vulnerable. The preliminary papillary layer is supplied arterially via the ciliary arteries, but venous outflow occurs via the central retinal vein. In contrast to the retinal vessels, the ciliary vessels have intense autonomic innervation and are affected by, for example, emotional stress to a greater extent. There are no arterioles in this layer, only long capillaries, which increases mechanical sensitivity. There is also the so-called “physiological defect” of the blood-brain barrier, which enables the vasoactive molecules direct access to smooth muscle cells and pericytes. This allows molecules, such as endothelin, to significantly reduce blood circulation in this region. All this explains why the mitochondria in this region are especially prone to oxidative damage.
Risk factors
We distinguish between risk factors that lead to an increase in ocular pressure (ocular hypertension) and risk factors that promote the development of GON (Figure 2).
Risk factors for ocular hypertension
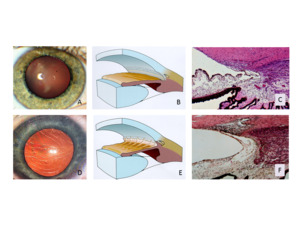
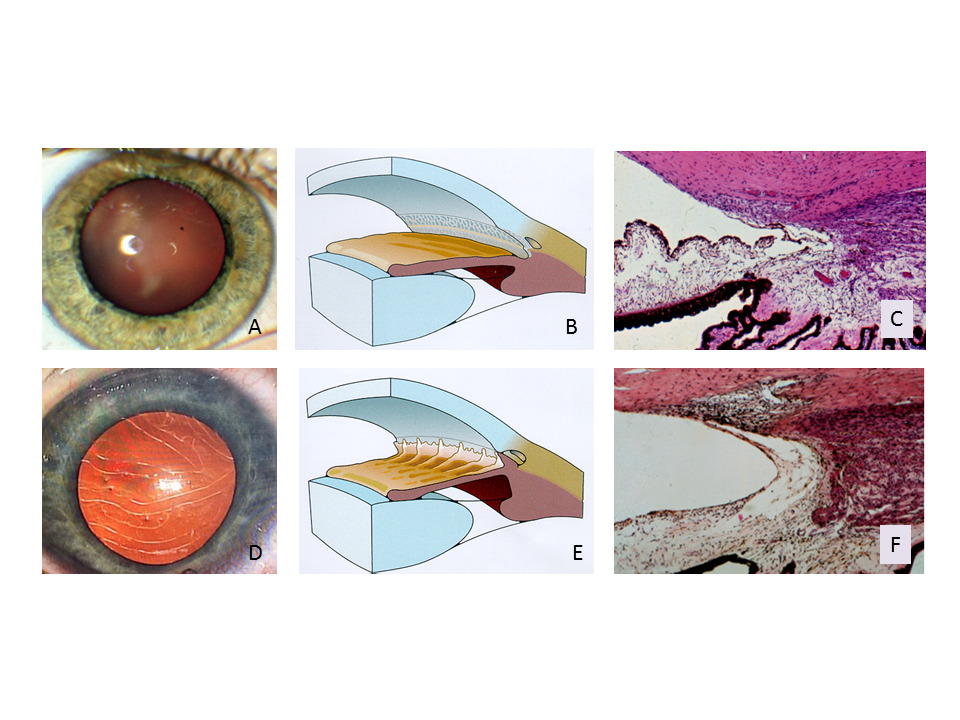
Intraocular pressure is elevated when the chamber angle is insufficiently developed (childhood glaucoma) (Figure 3), when the chamber angle is narrow and partially closed (narrow-angle glaucoma) or when it is totally closed (acute angle-closure glaucoma) (Figure 4).
Especially serious is the closure of the chamber angle resulting from neovascularization (hemorrhagic glaucoma) (Figure 5). However, even with an open chamber angle, intraocular pressure increases if the trabecular meshwork becomes blocked with blood or inflammatory material (secondary glaucoma) (Figure 5). Pigment (pigment dispersion syndrome) (Figure 6) or PEX material (pseudoexfoliation syndrome) can also cause blockage (Figure 7). However, by far the most common is primary open-angle glaucoma. With age, this leads to chronic remodeling of the trabecular meshwork, causing increased outflow resistance (Figure 8). Interestingly, all known arteriosclerosis risk factors are also risk factors for this tissue remodeling in the trabecular meshwork.
Risk factors for GON
Intraocular pressure
The most significant risk factor for GON is intraocular pressure. When the intraocular pressure increases, so does the probability of the development or progression of GON. Although intraocular pressure undoubtedly plays a role, the following must also be put into perspective: GON can develop at any intraocular pressure level, and increased intraocular pressure does not always lead to GON. For example, in Japan and Korea, more than 90% of glaucoma patients show no increase in intraocular pressure. It is therefore beyond doubt that other factors play a role. As a rule of thumb, the lower the intraocular pressure in cases where damage is identified, the more likely that other factors are involved. Today, three factors are the focus of discussion: low cerebrospinal fluid pressure, optic nerve compartment syndrome, and vascular disorders.
Cerebrospinal fluid (CSF) pressure
On average, the lumbar measurements of patients with normal-tension glaucoma (NTG) showed lower CSF pressure. It is postulated that the resulting greater pressure gradient in the lamina cribrosa contributes to glaucoma damage. However, as the relationships are so complex, several questions remain unresolved. For instance, there is only a limited correlation between lumbar CSF pressure and those around the optic nerve. Also, to a certain extent, intraocular pressure correlates with intracranial pressure. Additionally, association does not automatically equal causality. On average, patients with NTG not only have lower CSF pressure but also frequent arterial hypotension (a known risk factor for GON), which is particularly relevant for patients with impaired autoregulation of ocular blood flow.
Optic nerve compartment syndrome
Optic nerve compartment syndrome (ONCS), which was first described by H.E. Killer, occurs when free communication of cerebrospinal fluid (CSF) between the subarachnoid space of the optic nerve and the intracranial subarachnoid space is fully or partially interrupted. Measurable differences develop in the composition of the CSF and expansion of the optic nerve sheath, which is caused by the increased CSF pressure. The retinal venous pressure is usually also elevated. ONCS is frequently found in NTG patients. However, as it is often associated with Flammer syndrome (FS: see below), it may be difficult to differentiate if the damage is due to ONCS, FS, or both.
Ocular circulation
Since GON was first observed by Albrecht von Graefe more than 160 years ago, it was always suspected that blood circulation within the eye plays a role in the development and progression of GON. However, the study findings were contradictory. This only changed with the differentiation between unstable circulation and reduced circulation. Arteriosclerosis and its associated risk factors can potentially reduce blood circulation and increase the risk of elevated intraocular pressure. This makes them indirect (via intraocular pressure) risk factors for GON (Figure 2). However, intraocular pressure aside, atherosclerosis is only an insignificant risk factor for GON. For example, this can be observed by the fact that carotid stenosis alone presents no significant risk of GON. In turn, this means that GON is not solely a simple consequence of hypoxia. Rather, it is chronic oxidative stress that leads to glaucoma damage. A main cause of oxidative stress is an unstable oxygen supply. The oxygen concentration can fluctuate as a result of sleep apnea, but more commonly due to unstable blood circulation. Blood flow fluctuates when intraocular pressure fluctuates at a high level or when blood pressure fluctuates at a low level, exceeding the autoregulatory capacity from time to time (Figure 9). Blood circulation is more commonly and persistently unstable when autoregulation itself is disrupted. There are many factors that can influence autoregulation. A significant cause relating to glaucoma, and in particular NTG, is primary vascular dysregulation (PVD).2,3 PVD is the main vascular component of Flammer syndrome.
Flammer syndrome
Flammer syndrome (FS) describes the phenotype of people with a predisposition for an altered reaction of the blood vessels to stimuli such as cold, emotional stress, or high altitudes.2,4 Common symptoms are cold hands and/or feet, low blood pressure, longer time to fall asleep, reduced thirst, and increased sensitivity to smells, pain, vibrations, and certain types of medications. Common signs are quantitatively altered gene expression in the circulating lymphocytes, prolonged cessation of the blood flow following cold provocation (e.g., in the nail fold capillaries), reduced vascular dilation upon flicker stimulation, and – especially important for the development of GON – reduced autoregulation of the ocular blood flow. On average, retinal venous pressure is higher.2,5 Glaucoma patients with FS more frequently have visible astrocyte activation and optic disc hemorrhages. FS is more common in women than in men, in thin people than in obese people, in younger than older people, and in academics more than workers. It is rare in those who work outside. People with FS are generally ambitious and successful, with a tendency to be perfectionists. They also have a preference for some forms of exercise, such as jogging and cycling. FS is associated with NTG (Figure 2) but also with ocular vascular occlusions, retinitis pigmentosa, multiple sclerosis, tinnitus, or even sudden hearing loss. The role played by FS in other types of disease, such as breast carcinomas, is currently being investigated.
Epidemiology
Due to the lack of a uniform definition, we do not have accurate information on the prevalence of glaucoma in the general population. Approximately 2% of the population is affected, with older people far more commonly affected than young people. NTG and narrow-angle glaucoma are more common in women than in men. However, men suffer more commonly from pigment dispersion syndrome. Primary open-angle glaucoma is far more common in the sub-Saharan African population than in Europeans. In Europe, high tension glaucoma is the most common type, while in Asia it is NTG. Narrow-angle glaucoma is much more frequently found in India than in Europe. Pseudoexfoliation glaucoma is especially prevalent in Scandinavian countries.
Diagnostics
It is still recommended to carry out glaucoma screening when the patient is fitted with their first pair of reading glasses (earlier if there is a history of glaucoma in the family). The diagnostic instruments available, especially in the field of early-stage diagnostics, have improved greatly in recent years. However, the diagnostic core remains unchanged.
The following are paramount:
-
A good anamnesis;
-
Clinical examination, especially of the optic nerve head;
-
Perimetry if glaucoma is suspected.
The challenge for the ophthalmologist is, on the one hand, to identify GON relevant for the patient’s vision that requires treatment and, on the other, to avoid over-diagnosis and subsequent unnecessary treatments. After all, not every patient with a small amount of damage, or with damage only detectable by diagnostic instruments, will develop a significant visual field defect during their lifetime.
Since intraocular pressure can strongly fluctuate, patients suspected of having glaucoma require repeated testing at various times throughout the day.
If the development or progression of damage cannot be explained by intraocular pressure, then vascular diagnostics are recommended.
The following methods have been proven to be successful for us:
-
Targeted questioning relating to FS symptoms;
-
24-hour blood pressure monitoring, among others, to identify low levels;
-
Measurement of retinal venous pressure, among others, to estimate perfusion pressure;
-
Dynamic vessel analysis using flickering light to identify vascular dysregulation.
MRI is not necessary as standard, also not for NTG patients. However, it is indicated if:
-
Optic nerve atrophy is suspected to be non-glaucomatous;
-
The morphology of the optic nerve head does not correspond to visual field defects;
-
Visual acuity drops off at a relatively early stage;
-
There are notably large and unexplained differences between the right and left eye.
The use of ultrasound to search for optic nerve compartment syndrome or, if necessary, a cisternography is indicated if:
-
The neuroretinal rim is pale;
-
The visual field defects are greater than what would normally be expected based on the morphology of the optic nerve head;
-
The visual field defects are atypical.
Therapy
The first therapeutic approach is the reduction of intraocular pressure using eye drops, primarily prostaglandin analogs, carbonic anhydrase inhibitors, alpha-2 agonists, and beta blockers. Drug therapy can be supplemented with laser therapy. In the event of a poor intraocular pressure response, and, in particular, if the damage is progressive, surgery should be done to reduce the intraocular pressure. The surgical method applied depends mainly on the operating surgeon. If damage occurs together with preexisting low intraocular pressure or remains progressive despite reduced intraocular pressure, then other treatments should be considered, especially those that improve blood circulation in the eyes.
Although the various risk factors can be present independently of one another, the damage caused does not happen independently. For example, if autoregulation is disrupted, then the affected eye is unable to deal even with normal intraocular pressure and its fluctuation. Therefore, unstable blood circulation is usually the result. In theory, it is possible to either improve autoregulation or to reduce intraocular pressure so that blood flow regulation is not overly stressed. However, in practice, there are always limitations. On the one hand, it is not possible to reduce intraocular pressure at will without producing side effects, and on the other hand, improving autoregulation is not always simple. In addition, large controlled studies on the effects of vascular therapy on visual field prognosis are not yet available. This is mainly because there is no patent protection available for current treatment, and large companies are therefore not interested in conducting this type of study. The lack of final evidence does not rule out the efficacy of a vascular treatment. The avoidance of major falls in blood pressure, the improvement of blood circulation regulation, and the reduction of oxidative stress in the mitochondria have all been proven to be effective in the clinical setting.
Important for the practical setting
-
No uniform definition of glaucoma exists in the current literature. Today, the phenomenological definition is most commonly used.
-
The main risk factors for glaucoma damage are increased intraocular pressure, low perfusion pressure, and vascular dysregulation.
-
The activation of astrocytes and oxidative stress in the mitochondria of the affected axons, caused primarily by an unstable supply of oxygen, play a significant role in the development of glaucoma damage.
-
Glaucoma therapy depends on the constellation of risk factors. Most important are intraocular pressure reduction, optimization of the blood circulation, and protection of the mitochondria from oxidative stress.
Conflict of interest
None.
Disclosure statement
The authors have declared no personal or financial interests relating to this article.

_becomes_narrower.jpeg)


_can_also_cause_obstruction_of_the_trabecular_meshwork.tiff)

.jpeg)

_becomes_narrower.jpeg)


_can_also_cause_obstruction_of_the_trabecular_meshwork.tiff)

.jpeg)