Introduction
Coronavirus Disease 2019 (COVID-19) has widely spread all over the world since the beginning of 2020. It is highly contagious and may lead to acute respiratory distress or multiple organ failure in severe cases.1–3 The symptoms are manifested by form(s) of fever, cough, sore throat, breathlessness, fatigue, malaise, and may cause pneumonia and/or acute respiratory distress syndrome (ARDS).4 On 30th January 2020, the World Health Organization (WHO) declared the spread of COVID-19 to be a public emergency of international concern.4
As of 8th January 2021, 89,107,341 cases and 1,916,087 confirmed deaths due to COVID-19 have been reported globally according to Worldmeter COVID-19 Data.5
Although severe travel restrictions and social distancing measures helped to bring about a slowdown in spreading the disease, infections with SARS-CoV-2 are still on the rise in most countries.6
The disease is transmitted via inhalation or contact with virus-containing droplets. The incubation period ranges from two to fourteen days or even longer. The estimated fatality rate ranges from two to three percent. The virus can be detected in respiratory secretions by special molecular tests, such as polymerase chain reaction (PCR) based tests detecting specific sequences in the RNA genome of SARS-CoV-2.7 Elevated levels of C-reactive protein have been detected in the blood samples, while the white blood cell counts were considered in most cases to be normal. The computerized tomographic chest scan is usually abnormal even in some subjects with no symptoms or mild disease.8
Healthcare workers are facing challenges in reducing the severity and mortality of COVID‐19 across the world. Patients with severe COVID‐19 are generally treated in the hospital, with intensive care management. Patients with mild or non‐severe symptoms are treated symptomatically out of the clinic. However, there is an emerging challenge that a small subset of mild or non‐severe COVID‐19 patients develops into a severe disease course. Therefore, it is important to early identify and manage treatment for these patients to avoid tough complication conditions.
Reverse Transcription (RT)-PCR tests are considered the golden standard for detecting many viruses. Several tests are commercially available. Researchers at the Foundation for Innovative New Diagnostics, a nonprofit research center in Geneva, tested five COVID-19 RT-PCR tests and found that all five achieved 100% sensitivity on positive samples and at least 96% specificity on negative samples in a laboratory setting.9 In practice, testing conditions and processes are far from perfect, and leading to inaccurate results. It is still unclear what the real-world false positive rate is, but the clinical sensitivity of RT-PCR tests ranges from 66%–80%. That means nearly one in three infected people who are tested may receive a false negative result.
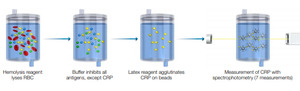
Clinical studies demonstrated that altered levels of some blood markers might be linked with the degree of severity and mortality of patients with COVID‐19.10–14 Of these clinical parameters, serum C‐reactive protein (CRP) has been found as an important marker that changes significantly in severe patients with COVID‐19.12 CRP is a type of protein produced by the liver that serves as an early marker of infection and inflammation.15 In blood, the normal concentration of CRP is less than 10 mg/L; however rises rapidly within 6–8 hours giving the highest peak in 48 hours from the disease onset.16 Its half‐life is about 19 hours,17 and its concentration decreases when the inflammatory stages end and the patient is healing. CRP preferably binds to phosphocholine expressed highly on the surface of damaged cells.18 This binding activates the classical complement pathway of the immune system and modulates the phagocytic activity to clear microbes and damaged cells from the organism.16 When the inflammation or tissue damage is resolved, CRP concentration falls, making it a useful marker for monitoring disease severity (Figure 1).16
The elevated CRP levels might be linked to the overproduction of inflammatory cytokines in severe patients with COVID‐19 or severe upper respiratory tract infection. Cytokines fight against the microbes, but it can damage lung tissue when the immune system becomes hyperactive. Thus, CRP production is induced by inflammatory cytokines and by tissue destruction in patients with COVID‐19. An elevated CRP level may be a valuable early marker in predicting the possibility of disease progression in non‐severe patients with COVID‐19, which can help health workers identify those patients at an early stage for early treatment. Besides, COVID‐19 patients with elevated CRP levels need close monitoring and treatment even though they did not develop symptoms to meet the criteria for the severe disease course. The CRP levels in patients with COVID‐19 who may progress from non‐severe to severe cases need to be further studied in large‐scale multicenter studies.
Since the onset of the outbreak, many pharmaceutical agents that could have efficacy against COVID-19 have been proposed. Various antiviral agents were included in the latest guidelines from the National Health Commission, including interferon, lopinavir/ritonavir, chloroquine phosphate, ribavirin, and arbidol.19 Angiotensin receptor blockers, such as losartan, have also been suggested for the treatment of COVID-19.20 Current treatment options are essentially supportive, as the role of antiviral agents is yet to be established. Preventive measures can be utilized to slow the spread of the infection, such as isolation of suspected cases or those with mild symptoms. Strict infection control measures should be taken at medical centers.
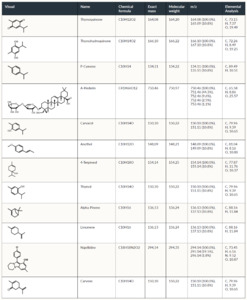
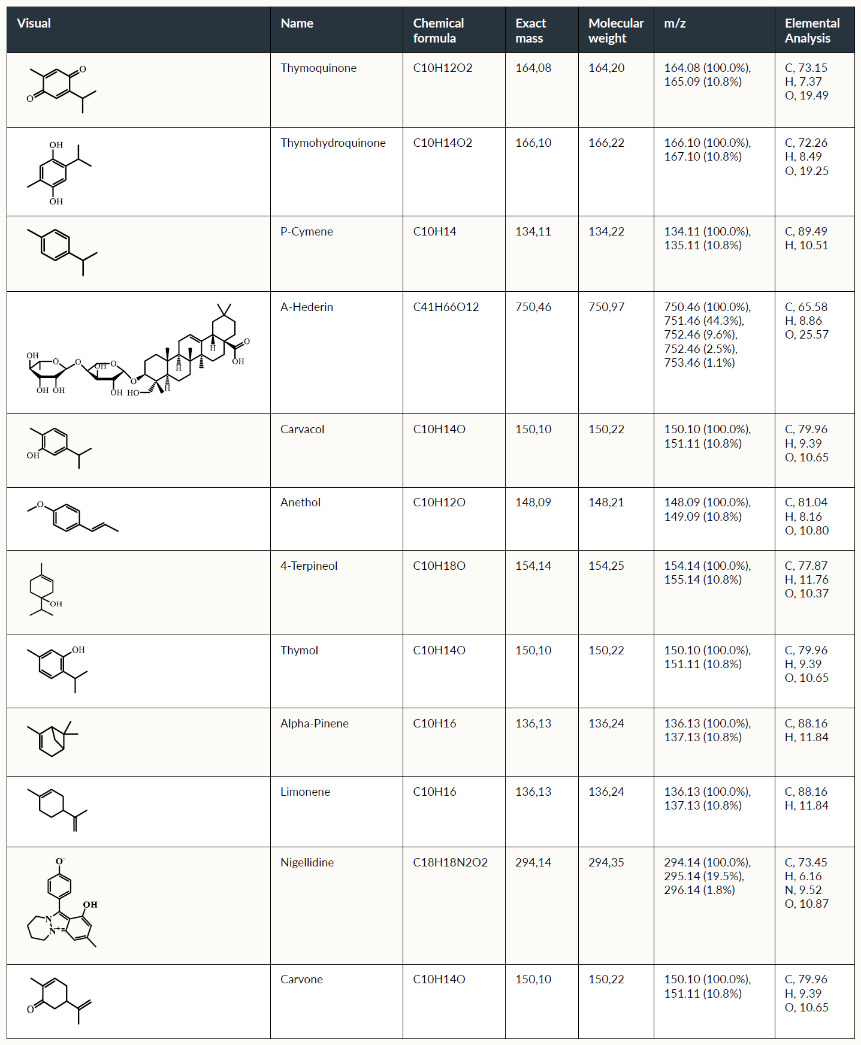
The chemical structure and properties of Nigella Sativa (N. sativa) are discussed in Fetian et al.21 and listed briefly in Table 1.
The work conducted in this case study is carried out in response to urgent needs to fight the nCoV-19 pandemic. It focuses and explores the potential characteristics of N. sativa in resisting many infectious diseases by modulating and boosting the strength of the immune system in an attempt to use it as part of a potential medical pharmaceutical.
Method
As the number of COVID-19 cases in Switzerland and elsewhere expands, healthcare providers were conducting drive-through COVID-19 screening testing to help to protect patients and healthcare workers from contracting the virus. Drive-through COVID-19 screening was commenced on 18th March 2020.
Patients were included in this case study if one or more of the following clinical symptoms were observed:
-
Symptoms of acute respiratory disease (e.g., coughing, sore throat, shortness of breath, chest pain)
-
Fever without other etiology
-
Sudden loss of the sense of smell and taste
-
In elderly individuals, acute confusion or deterioration in general health without other etiology
-
Muscle pain
-
Chills, and repeated shaking with chills
-
Headache
-
Nausea or vomiting
-
Diarrhea, fatigue, congestion, or runny nose
For this case study, only symptomatic patients where the swab has been done were asked to take NS.
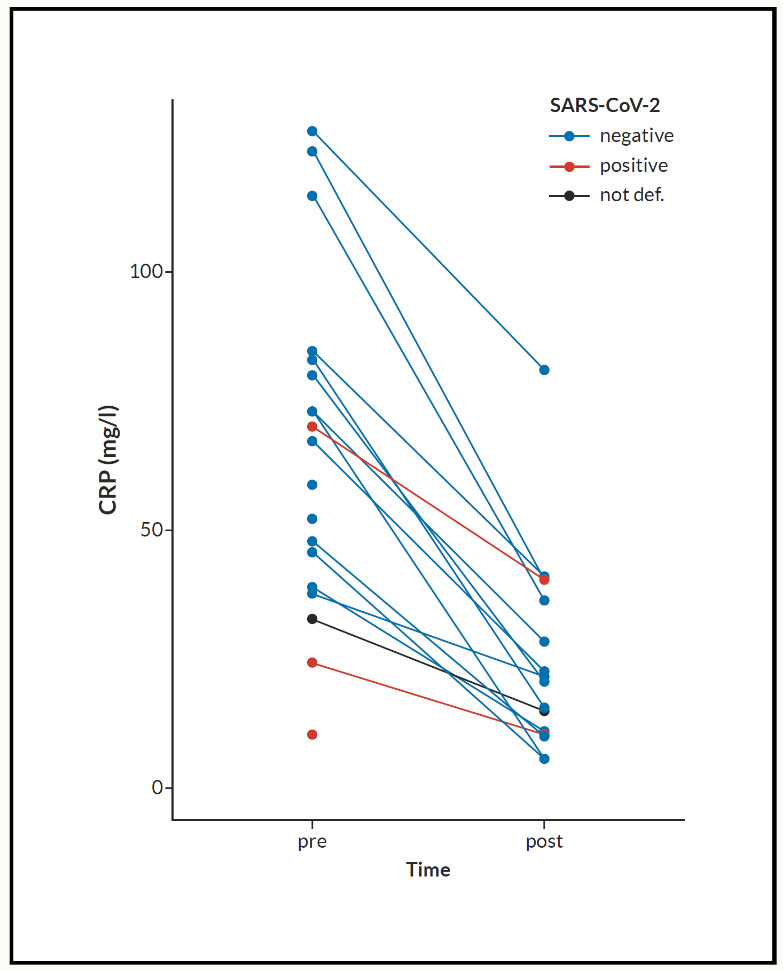
Measurement of CRP before and after the PCR test was offered to the symptomatic patients (Figure 1).
The results are reported by either a phone call or follow-up consultation a few days after the patient taking NS.
Laboratory analysis
Nasopharyngeal swab, SARS-CoV-2 Test procedure were performed in Dättwil with the diagnostic testing from the Lab enterprise Analytica from Zurich and with the Lab enterprise Risch from Brugg. The test procedure of both labs was an RT-PCR. Both labs used the Light Mix Modular SARS-CoV-2 from Roche.
The CRP test is performed in both centers using immunoturbidimetry with Microsemi from Horiba. Quality control is guaranteed by the Swiss lab standards.
N. sativa was suggested to the patient as a natural supplement due to the lack of possibilities for clinical examination of suspected cases in the light of pandemic disease and based on the parameter values as well as the history of present illness.
Clinical scenarios and results
The table (Table 2) shows the detailed information and the outcome in those patients with suspected or confirmed COVID-19. Therapy consists of a combined capsule of NS 500 mg and Vitamin E with or without iron oxide.
The outcome demonstrated a statistically significant improvement time (mean time: 3 days) in clinical symptoms and a reduction in measured CRP. According to the previous studies of N. sativa, no masking effect on the CRP response was reported when compared to immunosuppressants such as tocilizumab.22,23 Because of the shortage of logistic support and a high number of patient load, it was not possible to carry out CRP test measurement for every patient, and particularly those at the late stage of conducting this study. A proposed solution is to conduct a collaborative pilot clinical study using N. sativa on a larger population. This will explore and propagate its unique properties to COVID-19 treatment management.
Statistics
This is an analysis of the data of patient who are managed with Nigella sativa.
Description: Graphics
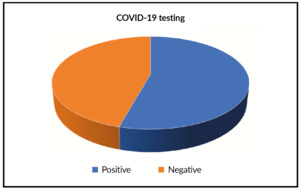
Comments: There is a significant decrease of CRP in all patients receiving Nigella sativa (NS). Both test t-test and Wilcoxon signed-rank test gives p-values <0.001. We included 35 patients (male 45.5%, female 54.5) (Figure 2). Age (mean+/-SD) was 51.79+/-17.32 years. CRP fall from 63.48+/-32.94 mg/L to 32.94+/-23.10 mg/L. SARS positive cases were 19 (55.9%) of whole tested patients (Figure 3). All patients had a significant reduction of their respective symptoms.
Discussion
Most of the medicinal properties of N. sativa were explored previously as an orally provided nutritional or dietary supplement of ground seeds in tablet or capsule form, oil extract in soft gel capsules, or as an aqueous extract in the form of fluid.24 Different combinations of N. sativa powder or extract with other vitamins such as vitamin E, are commonly offered as black seeds extract or black cumin oil to enhance natural physiological and immunological defense.
Extracts of N. sativa and specifically TQ, exhibited a broad antimicrobial range, including bacteria, viruses, and fungi. However, their effectiveness is dependent on the species of the target microorganisms.25 The mechanism of the antimicrobial effect of N. sativa has not been reported until now. Its antimicrobial property could be attributed to the active constituents particularly TQ and melanin.26 Their broad spectrum of activity may be the reason that the key processes of the organisms are affected.27
Endothelins are potent regulators of vascular tone, which also have mitogenic, apoptotic, and immunomodulatory properties.28,29 Three isoforms of endothelin have been identified to date, with endothelin-1 (ET-1) being considered the best route of study. ET-1 is classically considered a potent vasoconstrictor. However, in addition to the effects of ET-1 on vascular smooth muscle cells, the peptide is increasingly recognized as a proinflammatory cytokine.29,30 ET-1 causes platelet aggregation and plays a role in the increased expression of leukocyte adhesion molecules, the synthesis of inflammatory mediators contributing to vascular dysfunction. High levels of ET-1 are found in alveolar macrophages, leukocytes, and fibroblasts.30,31 Clinical and experimental data indicate that ET-1 is involved in the pathogenesis of sepsis,32,33 viral and bacterial pneumonia.34,35 Rickettsia conorii infections,36 Chagas disease,37,38 and severe malaria.39,40 A post-mortem histological analysis study of COVID-19 patients in Zurich revealed viral inclusion structures in endothelial cells. This histological study showed endotheliitis in many organs of the above-mentioned patients. In addition to that, endothelial dysfunction is a principal determinant of microvascular dysfunction by shifting the vascular equilibrium towards more vasoconstriction with subsequent organ ischemia, inflammation with associated tissue edema, and procoagulant state.41 A study has explained by their findings that the presence of viral elements within endothelial cells and an accumulation of inflammatory cells, with evidence of endothelial and inflammatory cell death. These findings suggest that SARS-CoV-2 infection facilitates the induction of endotheliitis in several organs as a direct consequence of viral involvement and of the host inflammatory response (Figure 5). In addition, induction of apoptosis and pyroptosis might have an important role in endothelial cell injury in patients with COVID-19.42 N. sativa has a reducing effect on oxidative like endothelin and it reduces the endothelin level in bronchoalveolar lavage and lung tissue.43
ACE2 has been identified as the functional receptor for SARS‐CoV.44 Li et al. showed that ACE2 can be immunoprecipitated by the S1 domain of the SARS‐CoV virus and that ACE2 can promote viral replication.
The antihypertensive effect of N. sativa is mediated by a reduction in cardiac oxidative stress and angiotensin-converting enzyme activity, an increase in cardiac heme oxygenase-1 activity, and prevention of plasma nitric oxide loss. Thus, N. sativa might be beneficial for controlling hypertension.45 It has an inhibitory effect of angiotensin-converting enzyme (ACE) that inhibits not only the conversion of angiotensin II from angiotensin I but also the bradykinase that degrades bradykinin (Figure 6). Bradykinin is generally a proinflammatory or an inflammatory mediator, which may inhibit viral replication.
SARS-CoV-2 has been shown to bind to angiotensin-converting enzyme 2 (ACE 2) to enter human cells by binding via the S protein on its surface. During infection, the S protein is cleaved into subunits S1 and S2. The S1 contains the receptor-binding domain (RBD), which allows coronaviruses to directly bind to the peptidase domain (PD) of ACE 2, and the S2 is believed to have a significant role in membrane fusion.46 Through the understanding of the ACE 2, it is expected to lead to producing antivirus or a vaccine that can block coronavirus infection by targeting ACE 2.
Take-Home Messages
-
Currently, there is a quite number of treatment options for viral infections with SARS-CoV-2 available. The majority of treatment options are of a supportive nature, and none is an absolute satisfactory therapeutic management. Thus, there are challenging further trends and trials for viral infection treatment management with SARS-CoV-2, with minimal or no side effects.
-
N. sativa exhibits the potential to be a distinguished drug for treating COVID-19 and different microbial infections. A pilot clinical study using N. sativa with big data and analytics is highly in demand based on the data presented above, which will propagate its unique properties to COVID-19 treatment management.
Disclosure statement
The authors have not declared any financial or personal connections in connection with this post.
Acknowledgments
The authors would like to thank Fabio Valeri for assistance with statistical analysis and careful review of the case report.