Introduction
Mucormycosis is an invasive fungal disease caused by fungi of the Mucorales order, representing a complex group of mold organisms. The most frequent genera that cause human infection are Rhizopus, Mucor, Lichtheimia, Cunninghamella, Apophysomyces and Rhizomucor spp.1
Mucormycosis is associated with a high mortality rate. Rapid recognition and early initiation of treatment with surgical fungal load reduction and antifungal therapy are crucial to improve survival.2,3 The mortality rate varies depending on various factors such as the immune status of the patient and the site of infection.4
In Europe, the most common underlying conditions in patients with mucormycosis are hematological malignancies, as described in the study of the European Confederation of Medical Mycology (ECMM) working group by Skiada et al. (2011).5 Diagnosis of mucormycosis in hematological patients, as of other invasive fungal diseases, is based on clinical, mycological and host criteria defined by the European Organization for Research and Treatment of Cancer/Mycoses Study Group (EORTC/MSG) Consensus Group.6,7 Other well-recognized predisposing conditions include solid organ transplantation, malignancy treatment, corticosteroid therapy, iron overload, major trauma, intravenous drug use and poorly controlled diabetes mellitus.8
Globally, diabetes is the most common underlying condition, estimated to be present in 36−40% of patients with mucormycosis, with marked geographical differences.1,4 A strong association is observed in India, where the prevalence of mucormycosis is nearly 70 times higher than the worldwide average.9,10 This is attributed to a high environmental burden of Mucorales, combined with a large number of individuals suffering from mostly poorly controlled or undiagnosed diabetes.10,11 In contrast, diabetes mellitus is less frequently encountered in Europe, reported in only 17% of the patients suffering from mucormycosis.5
Pulmonary mucormycosis has gained more attention in recent years, with a significant increase in cases associated with the COVID-19 pandemic, predominantly in Indian hospitals.12 In these patients diagnosed with COVID-19-associated mucormycosis (CAM), diabetes mellitus was also the most common underlying condition. Other associated factors were hypoxemia and partly improper use of glucocorticoids.
The clinical presentation of mucormycosis is characterized by a rapidly progressive infection with angioinvasion and thrombosis, resulting in tissue necrosis. The most common clinical manifestation in diabetic patients is rhino-orbital-cerebral mucormycosis, followed by the cutaneous and pulmonary forms. The latter is mostly seen in neutropenic patients.1,4,5,8
Here we present a case of isolated invasive pulmonary mucormycosis in a patient without underlying hematological disease but with poorly controlled diabetes mellitus.
Case presentation
Patient information
A 57-year-old female patient presented to her general practitioner with a 4-week history of coughing and hemoptysis accompanied by a weight loss of 12 kg. She denied having fever (without having taken her temperature), dyspnea or chest pain.
The patient had a medical history of insulin-dependent diabetes mellitus diagnosed at the age of 36. Over the last few months, blood glucose levels of up to 40 mmol/l (normal range: 4.3–6.4 mmol/l) were observed, despite regular therapy with long-acting insulin (insulin degludec) at a dose of 22 units per day. Other pre-existing comorbidities included arterial hypertension, hyperlipidemia and coronary heart disease. The patient was an active smoker with a cumulative 40 pack-years. She denied consumption of alcohol or other drugs. Her living environment involved a farm with chickens, ducks, turkeys, sheep and goats, and she kept cats and dogs as pets. There was no history of traveling abroad in the last few years, and no family member exhibited similar symptoms.
Due to her poor general condition, the patient was admitted to a peripheral hospital. A CT scan of the chest revealed a pulmonary infiltrate in the right lower lobe with central cavitation and mediastinal lymphadenopathy, raising suspicions of malignancy (Figure 1, A1 and A2). Since a bacterial abscess could not be excluded, an empirical antibiotic therapy with amoxicillin/clavulanic acid was initiated. For further evaluation, a bronchoscopy was planned and on day 8, the patient was referred to our tertiary-care hospital.
Clinical findings
Upon admission to our hospital, the patient presented in compromised condition with a body temperature of 39.2 °C. She was normotensive with signs of mild partial respiratory failure with an SpO2 of 88% in room air. Lung auscultation revealed diminished breath sounds over the right base. Further clinical examination showed no abnormal findings.
Diagnostic assessment
Laboratory analysis demonstrated elevated inflammatory markers, including leukocytosis (15 g/l; normal range: 3.5–10.0 g/l) with neutrophilic granulocytosis, C-reactive protein level of 120 mg/l (normal range: <10.0 mg/l), mild renal impairment, a hyporegenerative normochromic normocytic anemia with hemoglobin of 101 g/l (normal range: 120–160 g/l) and hypalbuminemia. Human immunodeficiency virus (HIV) screening and SARS-CoV2 PCR yielded negative results. The glycosylated hemoglobin level (HbA1c) was significantly elevated at 17.2% (normal range: 4.8–5.9%).
On the first day at our hospital, a follow-up chest CT was performed, showing a rapid progression within a week. The entire right lower lobe was consolidated with a central pulmonary abscess, and a new right-sided pleural effusion was found (Figure 1, B1 and B2). Bronchoscopy demonstrated a submucosal swelling of the right intermedial bronchus and the right lower lobe with subtotal stenosis (Figure 2). Under suspicion of malignancy, needle aspiration, transbronchial biopsy and bronchoalveolar lavage (BAL) were performed. The PCR panel for the most common respiratory pathogens (Respiratory Panel 2.1 from BIOFIRE®) yielded negative results. Further microbiological examination indicated a minimal growth of oral/pharyngeal flora (<10,000 CFU/ml) without any other significant bacterial or fungal growth. Mycobacteria staining was negative.
Cytological examination of the transbronchial needle aspiration showed no evidence of malignancy. However, neutrophilic granulocytosis, detritus and isolated filamentous fungi, which could not be further morphologically classified, were detected. In addition, fungal PCR from the BAL was performed. Aspergillus PCR was negative, but Mucorales-specific and panfungal PCR yielded positive results. Subsequent sequence analysis identified the PCR signal (internal transcribed spacer [ITS]) as Rhizopus arrhizus, with sequence identity to reference sequences 572/573 base pairs.13
Based on these findings, we diagnosed pulmonary mucormycosis of the right lower lobe in the context of poorly controlled diabetes mellitus. A complementary brain magnetic resonance imaging (MRI) ruled out cerebral and paranasal sinus involvement.
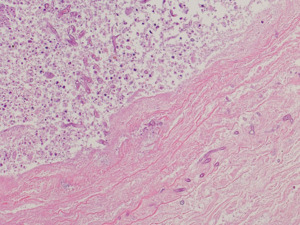
A follow-up bronchoscopy with bronchial biopsy and BAL confirmed Mucorales infection by histology (Figure 3) and positive molecular diagnosis for Mucorales/Rhizopus arrhizus. At this time, cultural bacterial growth of anaerobic flora and E. coli was detected. Rhizopus arrhizus could not be cultured preventing antifungal susceptibility testing.
Therapeutic intervention
Based on the ESCMID and ECMM guidelines,2 an intravenous antifungal therapy with liposomal amphotericin B (5 mg/kg daily) was started as soon as the diagnosis was made. In the absence of cerebral involvement, a high dose of 10 mg/kg daily was deemed unnecessary. Due to suspected bacterial superinfection, antibiotic therapy was continued and escalated to piperacillin/tazobactam.
An interdisciplinary team, including infectious diseases, pneumology, thoracic surgery and internal medicine, reached a consensus for surgical resection. This decision was made based on the extensive findings and reported hemoptysis as a sign of advanced fungal angioinvasion. Six days after admission to our hospital, video-assisted thoracoscopic surgery was performed with total resection of the right lower lobe and partial right parietal pleurectomy. Histologic examination of the lung tissue showed an angioinvasive filamentous fungal infection compatible with Mucorales with circumscribed necrosis and no involvement of the resected parietal pleura (Figure 4 and Figure 5).
The patient had a favorable postoperative course. The antibiotic therapy with piperacillin/tazobactam was discontinued after a total of 7 days. Antifungal therapy with liposomal amphotericin B was continued until the results of the intraoperative samples were obtained. Considering the angioinvasive growth, antifungal therapy was continued despite surgical resection in toto. After 2 weeks of treatment with liposomal amphotericin B, a step-down therapy to isavuconazole was considered appropriate. We started with a loading dose of 200 mg every 8 hours for the first 2 days, followed by isavuconazole 200 mg once daily. The therapy was initially administered intravenously and subsequently switched to peroral administration. Furthermore, management of the so far poorly controlled diabetes mellitus was intensified with adjustment of the insulin therapy.
Ten days after surgery, the patient was discharged home in a generally good health state.
Outcome
After discharge, the patient reported rapid clinical improvement, with the resolution of breathing difficulties or coughing. Her main complaint was a residual post-surgery scar pain, which persisted for almost 3 months.
The prescribed medication was taken regularly. As a side effect, the patient reported mild gastrointestinal symptoms in the form of diarrhea. She had frequent check-ups by an endocrinologist and initiation of an insulin pump therapy was planned.
A follow-up CT three months after the lobectomy showed regular postoperative findings without signs of persistent infection. Given the favorable clinical and radiological outcome, antifungal therapy with isavuconazole was stopped at that time. Four months after cessation of therapy there was no evidence of recurrence.
Discussion
We present the case of a Swiss patient with pulmonary consolidations, initially suspected as a malignancy based on clinical presentation and risk factors, but later identified as mucormycosis. Invasive pulmonary mucormycosis in patients without underlying hematological malignancy is rare in Europe and therefore often misinterpreted. Moreover, mucormycosis in the context of diabetes mostly manifests as rhino-orbital-cerebral infection, while lung involvement is less common. Yet, in the last years, cases with isolated pulmonary manifestations in diabetic patients were reported, mostly from India.14,15
In patients with pulmonary lesions and underlying risk factors like diabetes, it is essential to consider the possibility of invasive fungal disease in the initial differential diagnosis. The presence of a halo sign (central consolidation surrounded by ground glass opacities) and reversed halo sign (as in the initial CT scan in our case) should raise suspicion since they are often observed in invasive fungal diseases in the lower respiratory tract.2,6
The diagnosis of mucormycosis can be challenging. Non-invasive tests such as the 1,3-beta-d-glucan and galactomannan assay are not useful in diagnosing mucormycosis because beta-d-glucan and galactomannan are not present in the cell wall of Mucorales. Appropriate invasive diagnostic investigations should be promptly initiated. In our case, a bronchoscopy with transbronchial needle aspiration was rapidly performed. Histopathological findings of the bronchial biopsy showed the presence of fungi with associated tissue damage, consistent with invasive fungal disease. The mycological culture yielded no growth of fungi, but specific PCR tests verified the presence of fungal DNA, confirming the diagnosis and identifying the pathogen as Rhizopus arrhizus. This emphasizes the value of complementary use of PCR diagnostics to improve sensitivity and guide further treatment.13
We treated our patient according to international guidelines with aggressive surgical debridement and resection, accompanied by antifungal therapy.2 Immediate initiation of therapy with liposomal amphotericin B in the appropriate dosage is essential in the treatment of mucormycosis.16 Large reviews have shown a reduction in mortality with a combined approach.4,17
By presenting this case, we aim to raise awareness of mucormycosis in patients with uncontrolled diabetes mellitus and underscore the need for rapid diagnosis and initiation of treatment. Despite the natural aggressiveness of the disease, the patient’s outcome was favorable due to an appropriate diagnostic approach and combined treatment, along with intensification of therapy for the underlying predisposing condition. Interdisciplinary collaboration of all involved specialties (internal medicine, infectious disease, pneumology, microbiology, thoracic surgery, pathology and radiology) was crucial in achieving this goal.
Acknowledgments
We thank Max Schünemann for critically reading the manuscript.
Consent to participate
Written informed consent was obtained from the patient for publication of this case report.
Availability of data and materials
All patient data that support this case report are included in anonymized form in the published article.
Conflict of interest
The authors have declared that the study was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Funding
All authors have declared that no financial support was received from any organization for the submitted work.
Author contributions
NB and VB drafted the first version of this manuscript, whilst all authors intellectually contributed and revised this manuscript. MP assessed CT scans and provided images. Endoscopic interventions were done by MJH and KJ. KJ provided images. MW was the operating surgeon. SS carried out the histological analysis and provided images. DG supervised and analyzed microbiological methods. All authors read and approved the final manuscript.
_and_a2_(lung_kernel_coronal)_showed_a_lesion_in_the_right_lower_lob.jpeg)


_including_a_necro.png)
_and_a2_(lung_kernel_coronal)_showed_a_lesion_in_the_right_lower_lob.jpeg)


_including_a_necro.png)
